
Chemistry, 01.03.2021 22:20, jacobp0712
In the lab activity, the reaction rate was determined by the appearance of a product. However, the reaction rate can also be determined by the disappearance of a reactant. Rate =Δ[product]/Δt or rate−Δ[reactant]Δ t. In each situation below, you are given a rate measured by the appearance of one component of the reaction and are asked to predict the rate of appearance or disappearance of another component, based on logic and stoichiometric relationships.
For example, if the reaction is as follows:
A+2B⟶products
For every mole of A that is used, 2 moles of B are used so the rate of disappearance of B is twice the rate of the disappearance of A.
This may be expressed as:
rate=−Δ[B]/Δt=−2[A]/Δt , N2(g)+3H2(g)⟶2NH3(g)
The reaction rate is measured as 0.032 M NH3/s. Determine the rate of disappearance of N2 and the rate of disappearance of H2. Explain how you arrived at your answers.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
Do you know the correct answer?
In the lab activity, the reaction rate was determined by the appearance of a product. However, the r...
Questions in other subjects:




Mathematics, 04.12.2020 05:00


Mathematics, 04.12.2020 05:00

Mathematics, 04.12.2020 05:00




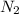 = 0.032 M/s
= 0.032 M/s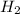 = 0.096 M/s
= 0.096 M/s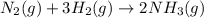
![-\frac{1d[N_2]}{dt}](/tpl/images/1158/2044/f2cf6.png)
![-\frac{1d[H_2]}{3dt}](/tpl/images/1158/2044/96c4e.png)
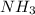 =
= ![\frac{1d[NH_3]}{2dt}](/tpl/images/1158/2044/ceb35.png)
![-\frac{1d[N_2]}{dt}=-\frac{1d[H_2]}{3dt}=\frac{1d[NH_3]}{2dt}](/tpl/images/1158/2044/4e2ff.png)
![-\frac{d[H_2]}{dt}=3\times 0.032M/s=0.096M/s](/tpl/images/1158/2044/7af2b.png)





