

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1


Chemistry, 23.06.2019 18:40, adibakawsar
Sound does not need a medium to travel is reflected when it bounces off a shiny surface is created by electric and magnetic fields moves in longitudinal waves
Answers: 1
Do you know the correct answer?
If 0.64 mol PCl5 is placed in a 1.0 L flask and allowed to reach equilibrium at a given temperature,...
Questions in other subjects:

Chemistry, 15.07.2019 16:30


Mathematics, 15.07.2019 16:30


Physics, 15.07.2019 16:30

Mathematics, 15.07.2019 16:30


History, 15.07.2019 16:30


Biology, 15.07.2019 16:30


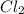 at equilibrium is 0.36 M
at equilibrium is 0.36 M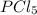 = 0.64 mole
= 0.64 mole 

![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/1158/2042/ffe89.png)





