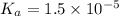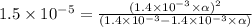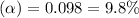Chemistry, 01.03.2021 21:50, andrejr0330jr
Calculate the percent dissociation of butanoic acid (C3H2CO2H) in a 1.4 mM aqueous solution of the stuff.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2

Do you know the correct answer?
Calculate the percent dissociation of butanoic acid (C3H2CO2H) in a 1.4 mM aqueous solution of the s...
Questions in other subjects:


Business, 13.12.2021 14:00

Mathematics, 13.12.2021 14:00

Mathematics, 13.12.2021 14:00

English, 13.12.2021 14:00



Mathematics, 13.12.2021 14:00

Mathematics, 13.12.2021 14:00

Mathematics, 13.12.2021 14:00


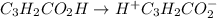
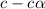
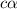
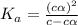
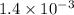 and
and 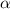 = dissociation constant
= dissociation constant