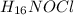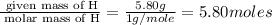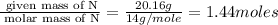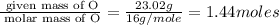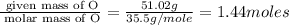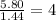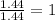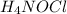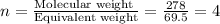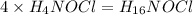Chemistry, 01.03.2021 21:50, dsaefong00
Hydroxylamine hydrochloride is a powerful reducing agent which is used as a polymerization catalyst. It contains 5.80 mass % H, 20.16 mass % N, 23.02 mass % O, and 51.02 mass % Cl. What is its empirical formula? Determine the molecular formula of the compound with molar mass of 278 g.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
Do you know the correct answer?
Hydroxylamine hydrochloride is a powerful reducing agent which is used as a polymerization catalyst....
Questions in other subjects:




English, 10.04.2020 22:58

Mathematics, 10.04.2020 22:58

Advanced Placement (AP), 10.04.2020 22:58

English, 10.04.2020 22:58



Physics, 10.04.2020 22:58