PLEASE HELP ASAP I WILL GIVE BRAINLIEST!
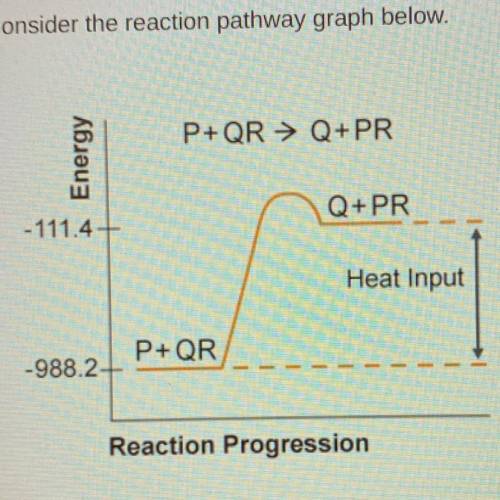
Consider the reaction pathway graph below.
The rate...

Chemistry, 01.03.2021 19:00, fixianstewart
PLEASE HELP ASAP I WILL GIVE BRAINLIEST!
Consider the reaction pathway graph below.
The rate increases by a factor of 9 when the concentration of A triples. The rate triples when the concentration of B triples. What is the new rate law for the reaction?
A) endothermic because Hrxn=-876.8 kJ
B) endothermic because Hrxn=876.8kJ
C)exothermic because Hrxn= -1099.6kJ
D) exothermic because Hrxn=1099.6kJ

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 06.04.2021 21:40

English, 06.04.2021 21:40



Mathematics, 06.04.2021 21:40


Mathematics, 06.04.2021 21:40









