
Chemistry, 01.03.2021 18:50, emmanuelmashao6704
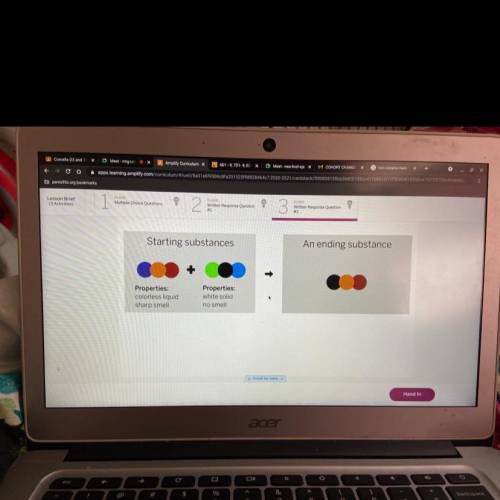
A chemist mixed two substances together: a colorless liquid with a strong smell and a white solid with no smell. The
substances' repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the
results and found two substances. One ending substance had the repeating group of atoms shown above on the right.
Is the ending substance the same substance as the colorless liquid? What happened to the atoms of the starting
substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2
Do you know the correct answer?
A chemist mixed two substances together: a colorless liquid with a strong smell and a white solid wi...
Questions in other subjects:


Chemistry, 30.08.2019 04:30




Chemistry, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Health, 30.08.2019 04:30







