
Chemistry, 01.03.2021 18:30, Matseleng3775
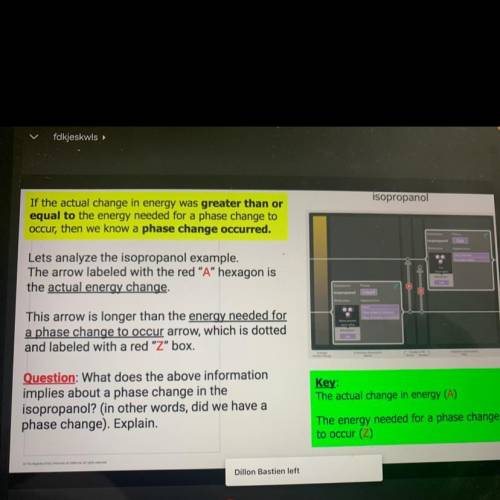
If the actual change in energy was greater than o
equal to the energy needed for a phase change to
occur, then we know a phase change occurred.
Lets analyze the isopropanol example,
The arrow labeled with the red "A" hexagon is
the actual energy change.
This arrow is longer than the energy needed for
a phase change to occur arrow, which is dotted
and labeled with a red "Z" box.
Question: What does the above information
implies about a phase change in the
isopropanol? (in other words, did we have a
phase change). Explain.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3


Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
Do you know the correct answer?
If the actual change in energy was greater than o
equal to the energy needed for a phase change to<...
Questions in other subjects:


Chemistry, 15.07.2019 11:30

English, 15.07.2019 11:30


English, 15.07.2019 11:30

Geography, 15.07.2019 11:30

History, 15.07.2019 11:30



History, 15.07.2019 11:30






