Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 19:10, aamu15
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2
Do you know the correct answer?
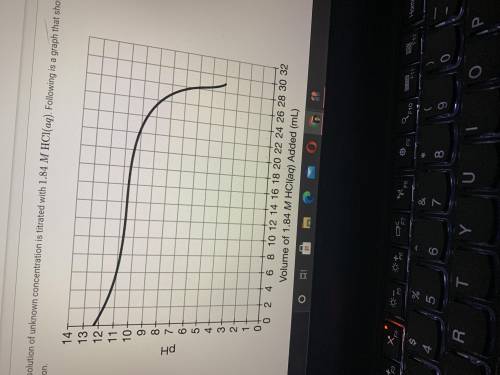
If 28.25mL of 1.84MHCl(aq) was required to reach the equivalence point, calculate the concentration...
Questions in other subjects:

Mathematics, 15.12.2020 01:00



Physics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

English, 15.12.2020 01:00

Medicine, 15.12.2020 01:00