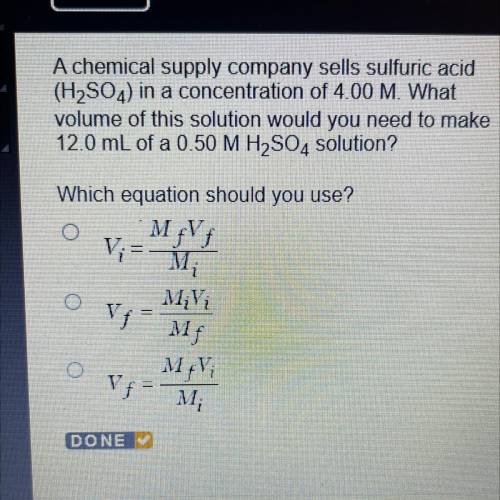
A chemical supply company sells sulfuric acid
(H2SO4) in a concentration of 4.00 M. What
volu...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Health, 13.12.2021 20:40

English, 13.12.2021 20:40

Spanish, 13.12.2021 20:40



Health, 13.12.2021 20:40

Chemistry, 13.12.2021 20:40

Chemistry, 13.12.2021 20:40

Mathematics, 13.12.2021 20:40