
Chemistry, 01.03.2021 04:10, krystalhurst97
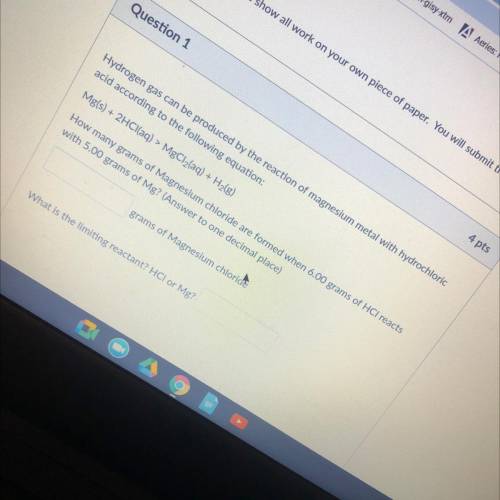
Hydrogen gas can be produced by the reaction of magnesium metal with hydrochloric
acid according to the following equation:
Mg(s) + 2HCl(aq) > MgCl2(aq) + H2(g)
How many grams of Magnesium chloride are formed when 6.00 grams of HCl reacts
with 5.00 grams of Mg? (Answer to one decimal place)
grams of Magnesium chloride
What is the limiting reactant? HCI or Mg?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 22:30, vhife4901
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
Do you know the correct answer?
Hydrogen gas can be produced by the reaction of magnesium metal with hydrochloric
acid according to...
Questions in other subjects:





Biology, 10.02.2020 08:23



Business, 10.02.2020 08:24

Mathematics, 10.02.2020 08:24

Mathematics, 10.02.2020 08:24






