
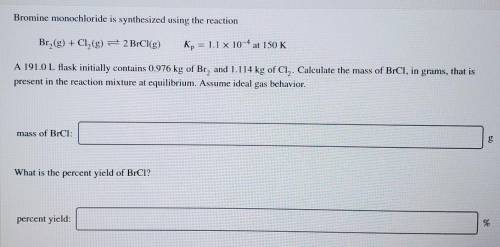
Bromine monochloride is synthesized using the reaction Br2(g) + Cl2(g) = 2 BrCl(g) Kp = 1.1 x 10^-4 at 150 K A 191.0 L flask initially contains 0.976 kg of Br2 and 1.114 kg of Cl. Calculate the mass of BrCl2 in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1


Chemistry, 23.06.2019 06:40, Taylor73836
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1

Chemistry, 24.06.2019 02:30, duttonsteven45
What is the oxidation number of o in po4^3-? a. 0 b. -1 c. -2 d. -3 e. none of the above
Answers: 1
Do you know the correct answer?
Bromine monochloride is synthesized using the reaction Br2(g) + Cl2(g) = 2 BrCl(g) Kp = 1.1 x 10^-4...
Questions in other subjects:




English, 16.03.2020 06:00

Mathematics, 16.03.2020 06:00


History, 16.03.2020 06:00


Mathematics, 16.03.2020 06:00






