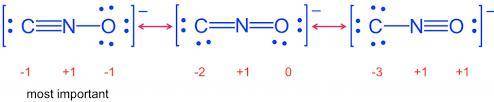Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
Do you know the correct answer?
Draw Lewis structures for the fulminate ion including possible resonance forms.
Draw the molecule b...
Questions in other subjects:


English, 09.02.2021 20:00

Mathematics, 09.02.2021 20:00

Health, 09.02.2021 20:00

Mathematics, 09.02.2021 20:00

Mathematics, 09.02.2021 20:00



Social Studies, 09.02.2021 20:00

Computers and Technology, 09.02.2021 20:00