
Chemistry, 27.02.2021 15:10, jadadugas4418
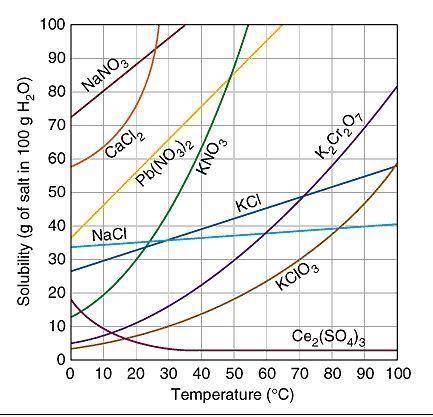
Based on the table above, which amount of a compound dissolved in 100 grams of water at the stated temperature represents a system at equilibrium?
Select one:
a. 40 g NaCl at 70 degrees Celsius
b. 20 g KClO3 at 70 degrees Celsius
c. 80 g NaNO3at 10 degrees Celsius
d. 80 g Pb(NO3)2 at 30 degrees Celsius

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1
Do you know the correct answer?
Based on the table above, which amount of a compound dissolved in 100 grams of water at the stated t...
Questions in other subjects:


Mathematics, 28.10.2020 07:50

Mathematics, 28.10.2020 07:50

Social Studies, 28.10.2020 07:50



History, 28.10.2020 07:50



Chemistry, 28.10.2020 07:50







