
Chemistry, 27.02.2021 14:10, kris22elizondop9v1bb
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸
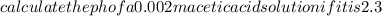

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
Do you know the correct answer?
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸...
Questions in other subjects:

Mathematics, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10




Mathematics, 27.04.2021 20:10

Chemistry, 27.04.2021 20:10







