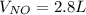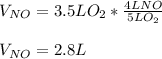Chemistry, 27.02.2021 08:00, georgesarkes12
4NH3(g) + 5O2(g) -> 4NO(g) +6H20(g) If 3.5 L of oxygen gas at STP react with an excess amount of ammonia, how many liters of nitrogen monoxide will be produced

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
Do you know the correct answer?
4NH3(g) + 5O2(g) -> 4NO(g) +6H20(g) If 3.5 L of oxygen gas at STP react with an excess amount of...
Questions in other subjects:


Chemistry, 02.06.2021 09:20



History, 02.06.2021 09:20

History, 02.06.2021 09:20


Mathematics, 02.06.2021 09:20

Mathematics, 02.06.2021 09:20