
An equilibrium mixture in a 16.2 L container at 712 K contains 0.218 mol NH4I(s), 0.189 M NH3 and 0.116 M HI. What will be the concentrations of the two gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at constant temperature to a volume of 6.79 L

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1

Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2
Do you know the correct answer?
An equilibrium mixture in a 16.2 L container at 712 K contains 0.218 mol NH4I(s), 0.189 M NH3 and 0....
Questions in other subjects:



Arts, 08.07.2020 07:01

Mathematics, 08.07.2020 07:01

Mathematics, 08.07.2020 07:01

Mathematics, 08.07.2020 07:01

Mathematics, 08.07.2020 07:01

Mathematics, 08.07.2020 07:01


History, 08.07.2020 07:01


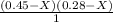 ----- ( 1 )
----- ( 1 ) 



