
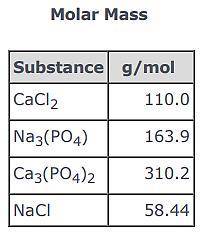
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass table above to answer the following question.
Suppose 163.9 g of Na3(PO4) reacted with sufficient CaCl2 in solution to actually yield 116 g of Ca3(PO4)2(s) . What is the percent yield of Ca3(PO4)2(s)?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 12:30, ritahastie7533
Atriple covalent bond involves two atoms sharing three pairs of electrons. true false
Answers: 2
Do you know the correct answer?
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass...
Questions in other subjects:

Mathematics, 06.08.2021 18:30














