Question 1 (1 point)
Above is a solubility curve for KNO3.
Solubility has nothing to do...

Chemistry, 26.02.2021 02:00, hectorgonzalejr333
Question 1 (1 point)
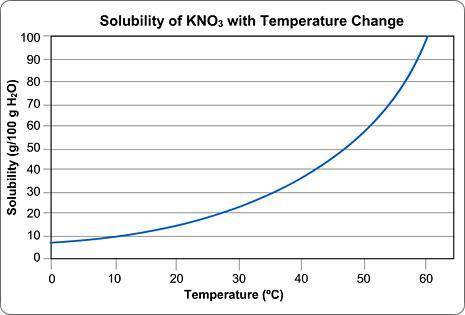
Above is a solubility curve for KNO3.
Solubility has nothing to do with the speed of dissolving; it's a measure of how much salt will dissolve at a given temperature.
The y-axis of the graph shows you how much KNO3 will dissolve in 100 g of water. In other words, it tells you the maximum amount of solute that will dissolve at different temperatures.
The x-axis tells you the minimum temperature needed to dissolve different amounts of KNO3 in 100 g of water.
Approximately how many grams of KNO3 will dissolve in 100 g water at 0 degrees Celsius?
Type in the number only; no units. Round your answer to the nearest whole number.
Question 1 options:
Question 2 (1 point)
Approximately what is the minimum temperature needed to dissolve 10 g of KNO3 in 100 g H2O?
Round your answer to the nearest whole number and type in the number only; no units.
Question 2 options:
Question 3 (1 point)
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after you've dissolved about 55 g of KNO3, you can't dissolve any more; it just sinks to the bottom.
Approximately what is the temperature of the water?
Round your answer to the nearest whole number and submit the number only; no units.
Question 3 options:
Question 4 (1 point)
Saved
Increasing the temperature of the water will increase the amount of salt that it can dissolve.
Question 4 options:
True
False
Question 5 (1 point)
Saved
A solubility curve identifies how fast a solute will dissolve at different temperatures.
Question 5 options:
True
False
Question 6 (1 point)
Saved
This solubility curve indicates that at higher temperatures KNO3 will dissolve faster.
Question 6 options:
True
False
Question 7 (1 point)
Saved
A solubility curve tells you the minimum temperature needed to dissolve an amount of solute.
Question 7 options:
True
False

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:30, cashkidd2200
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Computers and Technology, 16.11.2019 00:31















