
Chemistry, 25.02.2021 19:20, tyairamifflin2411
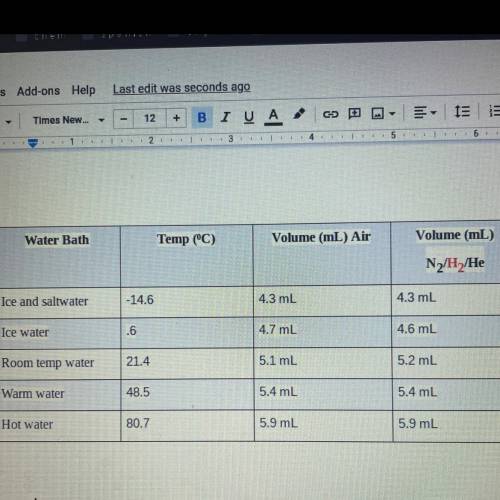
Calculations:
1. The actual value for absolute zero in degrees Celsius is –273.15. Use the formula below
to determine your percent error for both gas samples.
Jexperimental value - actual valuel x 100
actual value
2. If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in
each syringe? (Hint: Choose one volume and temperature pair from your data table to use
in your ideal gas law calculation.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Vicky22Shz
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
Do you know the correct answer?
Calculations:
1. The actual value for absolute zero in degrees Celsius is –273.15. Use the formula...
Questions in other subjects:


Chemistry, 30.11.2020 21:50


English, 30.11.2020 21:50


Chemistry, 30.11.2020 21:50


History, 30.11.2020 21:50


Mathematics, 30.11.2020 21:50






