
Chemistry, 25.02.2021 18:20, alejandr1872913
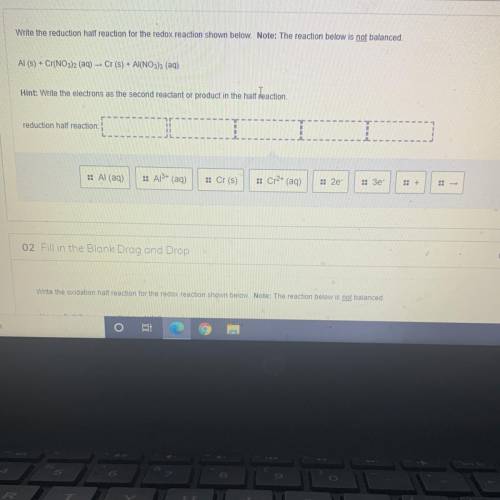
Write the reduction half reaction for the redox reaction shown below. Note: The reaction below is not balanced.
Al(s) + Cr(NO3)2 (aq) – Cr(s) + Al(NO3)3 (aq)
Hint: Write the electrons as the second reactant or product in the half reaction.
reduction half reaction:
:: Al (aq)
:: Al3+ (aq)
:: Cr(s)
:: Cr2+ (aq)
:: 2e
:: Зе-

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, Bryanguzman2004
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
Do you know the correct answer?
Write the reduction half reaction for the redox reaction shown below. Note: The reaction below is no...
Questions in other subjects:




Social Studies, 02.07.2019 09:30

Mathematics, 02.07.2019 09:30

Mathematics, 02.07.2019 09:30

Mathematics, 02.07.2019 09:30

Mathematics, 02.07.2019 09:30

Mathematics, 02.07.2019 09:30






