
Chemistry, 25.02.2021 06:30, diamondk2019
HELP
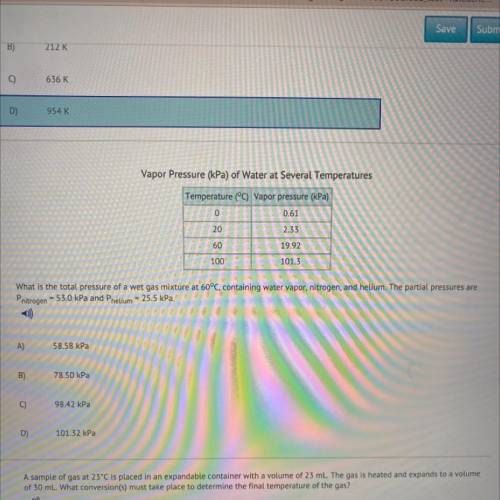
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and helium. The partial pressures are
Pnitrogen - 53.0 kPa and Phelium = 25.5 kPa.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1


Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
Do you know the correct answer?
HELP
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and...
Questions in other subjects:



Social Studies, 24.10.2021 01:00



Biology, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Business, 24.10.2021 01:00

Biology, 24.10.2021 01:00






