
Chemistry, 25.02.2021 01:00, bazsinghnagoke
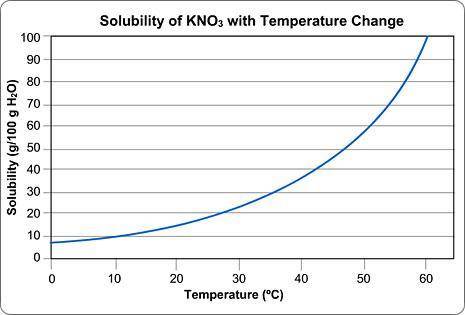
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after you've dissolved about 55 g of KNO3, you can't dissolve any more; it just sinks to the bottom.
Approximately what is the temperature of the water?
Round your answer to the nearest whole number and submit the number only; no units.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
Do you know the correct answer?
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after...
Questions in other subjects:


Mathematics, 25.06.2019 20:30

Mathematics, 25.06.2019 20:30


Health, 25.06.2019 20:30










