
Chemistry, 24.02.2021 22:10, ftbluedevil
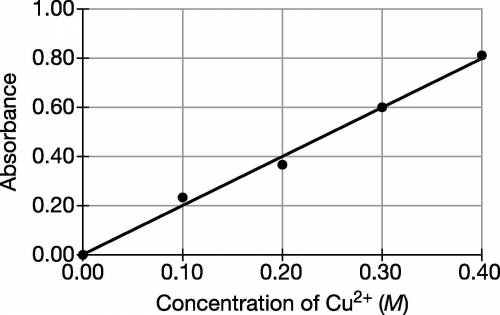
A student is given a sample of CuSO4(s) that contains a solid impurity that is soluble and colorless. The student wants to determine the amount of CuSO4 in the sample and decides to use a spectrophotometer. First, the student prepares a calibration graph by measuring the absorbances of CuSO4(aq) solutions of known concentrations. The graph is shown below. (a) The student dissolves the entire impure sample of CuSO4(s) in enough distilled water to make 100.mL of solution. Then the student measures the absorbance of the solution and observes that it is 0.30. Determine the concentration of CuSO4(aq) in the solution.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
Do you know the correct answer?
A student is given a sample of CuSO4(s) that contains a solid impurity that is soluble and colorless...
Questions in other subjects:

English, 25.03.2021 05:50

History, 25.03.2021 05:50


Mathematics, 25.03.2021 05:50

Chemistry, 25.03.2021 05:50

Mathematics, 25.03.2021 05:50


Chemistry, 25.03.2021 05:50

Advanced Placement (AP), 25.03.2021 05:50







