
Chemistry, 24.02.2021 21:30, CameronVand21
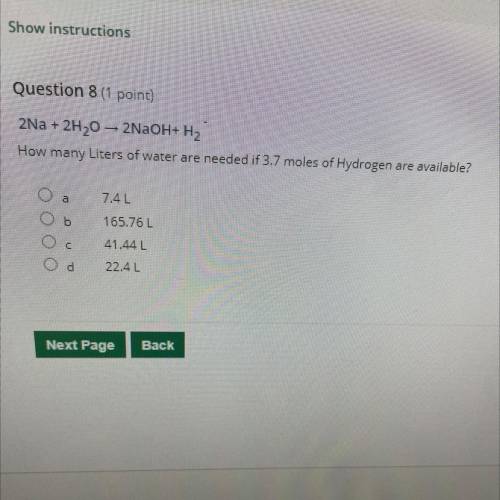
2Na + 2H20 – 2NaOH+H2 How many Liters of water are needed if 3.7 moles of Hydrogen are available?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 03:30, ilizzy1224
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3
Do you know the correct answer?
2Na + 2H20 – 2NaOH+H2
How many Liters of water are needed if 3.7 moles of Hydrogen are available?
<...
Questions in other subjects:



Mathematics, 01.12.2020 19:50

Mathematics, 01.12.2020 19:50

History, 01.12.2020 19:50

Computers and Technology, 01.12.2020 19:50

English, 01.12.2020 19:50









