
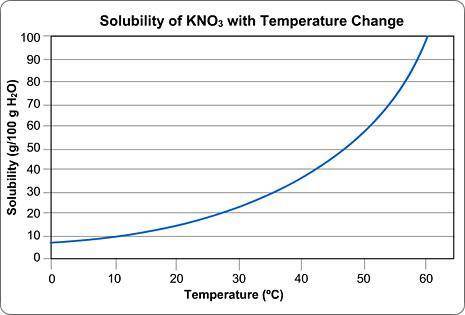
Above is a solubility curve for KNO3.
Solubility has nothing to do with the speed of dissolving; it's a measure of how much salt will dissolve at a given temperature.
The y-axis of the graph shows you how much KNO3 will dissolve in 100 g of water. In other words, it tells you the maximum amount of solute that will dissolve at different temperatures.
The x-axis tells you the minimum temperature needed to dissolve different amounts of KNO3 in 100 g of water.
Approximately how many grams of KNO3 will dissolve in 100 g water at 0 degrees Celsius?
Type in the number only; no units. Round your answer to the nearest whole number.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
Above is a solubility curve for KNO3.
Solubility has nothing to do with the speed of dissolving; it...
Questions in other subjects:

Mathematics, 14.07.2021 22:10

Mathematics, 14.07.2021 22:10

Chemistry, 14.07.2021 22:10



English, 14.07.2021 22:10

Business, 14.07.2021 22:10


Mathematics, 14.07.2021 22:10

Mathematics, 14.07.2021 22:10






