
Chemistry, 24.02.2021 05:20, lollipopboo
ASAP ASAP PLEASE PLEASE HELP HELP
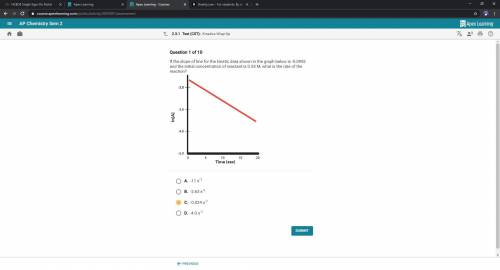
If the slope of line for the kinetic data shown in the graph below is -0.0952 and the initial concentration of reactant is 0.25 M, what is the rate of the reaction?


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ilizzy1224
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3


Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
Do you know the correct answer?
ASAP ASAP PLEASE PLEASE HELP HELP
If the slope of line for the kinetic data shown in the graph belo...
Questions in other subjects:






Chemistry, 05.05.2020 18:12




History, 05.05.2020 18:12






