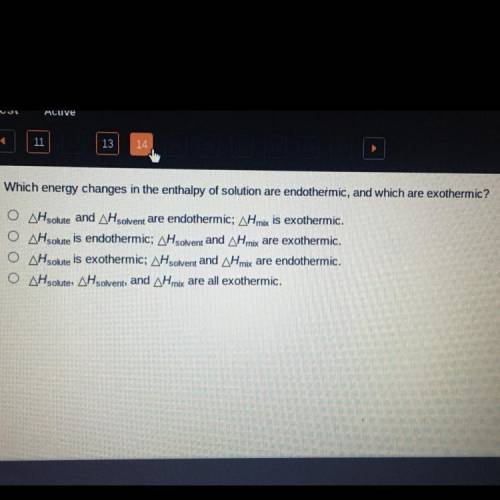
Which energy changes in the enthalpy of solution are endothermic, and which are exothermic?
...


Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2


Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Mathematics, 21.01.2021 17:00

English, 21.01.2021 17:00


Mathematics, 21.01.2021 17:00

Mathematics, 21.01.2021 17:00

History, 21.01.2021 17:00


Arts, 21.01.2021 17:00