
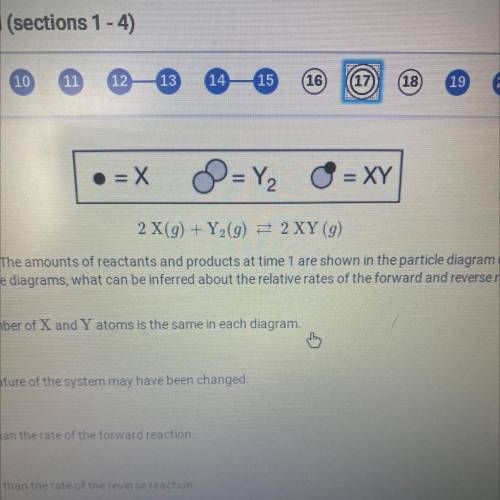
2 X(9) + Y (9) = 2XY (9)
A reversible reaction is represented by the equation above. The amounts of reactants and products at time 1 are shown in the particle diagram on the left. The particle diagram on the right shows the
amounts of reactants and products at time 2. Based on the diagrams, what can be inferred about the relative rates of the forward and reverse reactions between time 1 and time 2?
a. nothing can be inferred because the total number of X and Y atoms is the same
each diagram
b. Nothing can be inferred because the temperature of the system may have been changed
c. The rate of the reverse reaction is greater than the rate of the forward reaction
d. The rate of the forward reaction is greater than the rate of the reverse reaction.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, Angelanova69134
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
Do you know the correct answer?
2 X(9) + Y (9) = 2XY (9)
A reversible reaction is represented by the equation above. The amounts of...
Questions in other subjects:

Mathematics, 10.11.2020 21:50

Mathematics, 10.11.2020 21:50


Health, 10.11.2020 21:50

Mathematics, 10.11.2020 21:50

English, 10.11.2020 21:50


Chemistry, 10.11.2020 21:50


Biology, 10.11.2020 21:50






