
Chemistry, 23.02.2021 22:30, arnold2619
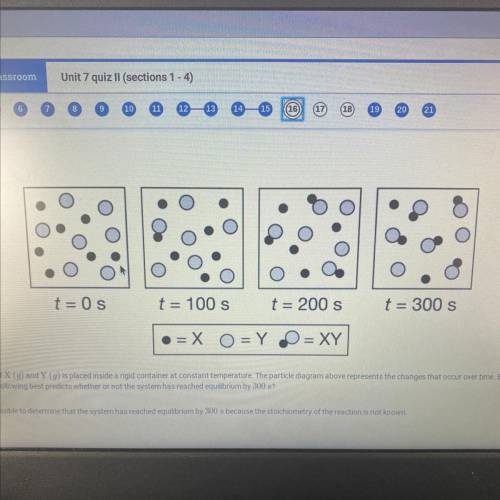
An equimolar mixture of X (g) and Y (g) is placed inside a rigid container at constant temperature. The particle diagram above represents the changes that occur over time. Based on the particle
diagram, which of the following best predicts whether or not the system has reached equilibrium by 300 s?
a. It is not possible to determine that the system has reached equilibrium by 300 s because the stoichiometry of the reaction is not known.
b. it is not possible to determine that the system has reached equilibrium by 300 s because the amounts of X, Y, and XY have continued to change
c. The system has reached equilibrium by 300 s because the rate of formation of XY is constant
d. The system has reached equilibrium by 300 s because the rates of consumption of X and Y are equal

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
Do you know the correct answer?
An equimolar mixture of X (g) and Y (g) is placed inside a rigid container at constant temperature....
Questions in other subjects:

English, 11.10.2019 18:20

Mathematics, 11.10.2019 18:20

English, 11.10.2019 18:20


Biology, 11.10.2019 18:20

History, 11.10.2019 18:20


Computers and Technology, 11.10.2019 18:20


English, 11.10.2019 18:20






