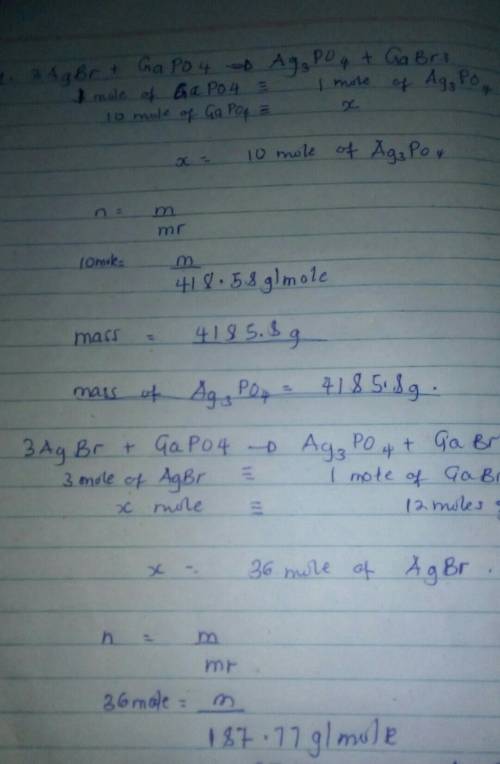Chemistry, 23.02.2021 21:20, aljalloh94
3AgBr + GaPO4 = Ag3PO4 + GaBr3
1. How many grams of Ag3PO4 are produced from the use of 10.0 moles of GaPO4?
2. How many grams of AgBr are needed to produce 12 moles of GaBr3?
3. How many grams of GaBr3 are produced from the reaction of 15 moles of GaPO4?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2


Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
Do you know the correct answer?
3AgBr + GaPO4 = Ag3PO4 + GaBr3
1. How many grams of Ag3PO4 are produced from the use of 10.0 moles...
Questions in other subjects:




Social Studies, 25.11.2021 08:00

Chemistry, 25.11.2021 08:00

Spanish, 25.11.2021 08:00


Biology, 25.11.2021 08:00


History, 25.11.2021 08:00