
Chemistry, 23.02.2021 09:40, amycressey1970
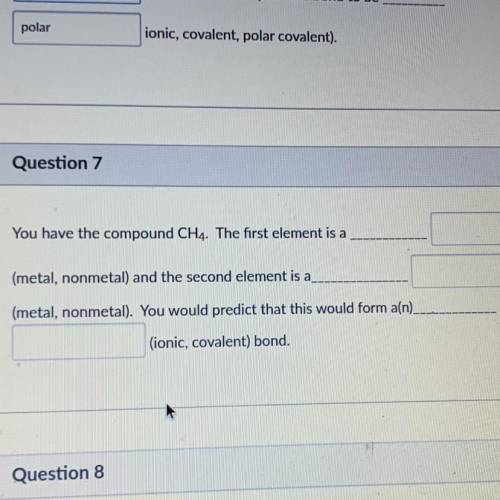
You have the compound CH4. The first element is a
(metal, nonmetal) and the second element is a
(metal, nonmetal). You would predict that this would form a(n).
(ionic, covalent) bond.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, leilanimontes714
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Do you know the correct answer?
You have the compound CH4. The first element is a
(metal, nonmetal) and the second element is a
Questions in other subjects:

Health, 17.06.2020 18:57

English, 17.06.2020 18:57


Mathematics, 17.06.2020 18:57




Physics, 17.06.2020 18:57







