
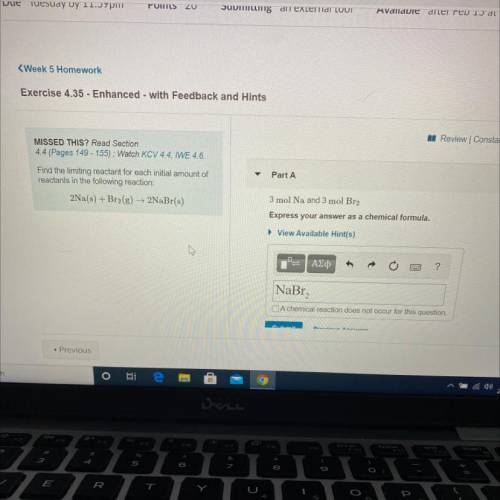
Find the limiting reactant for each initial amount of
reactants in the following reaction: 2Na(s) + Br2(g) + 2NaBr(s)
Part A: 3 mol Na and 3 mol Br2
Part B: 1.6 mol Na and 1.2 mol Br2
Part C: 2.5 mol Na and 1 mol Br2
Part D: 12.9 mol Na and 6.9 mol Br2
Express your answers as a chemical formula.
Please help! will give brainliest

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
Do you know the correct answer?
Find the limiting reactant for each initial amount of
reactants in the following reaction: 2Na(s) +...
Questions in other subjects:

Mathematics, 14.12.2020 20:30



Mathematics, 14.12.2020 20:30

Physics, 14.12.2020 20:30


Mathematics, 14.12.2020 20:30


Advanced Placement (AP), 14.12.2020 20:30

Mathematics, 14.12.2020 20:30






