compute the theoretical yield of the product (in

Chemistry, 23.02.2021 09:10, marissagirl9893
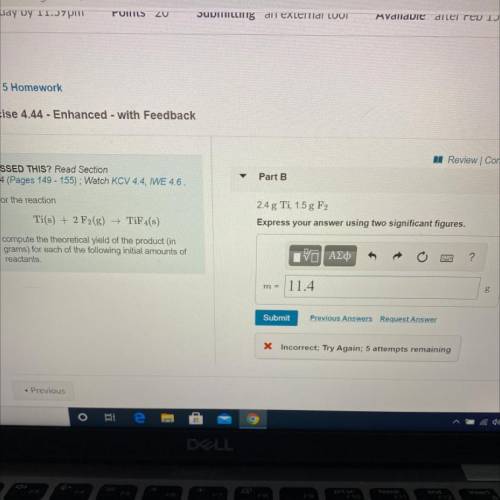
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
grams) for each of the following initial amounts of
reactants.
2.4 g Ti, 1.5 g F2
Express your answer using two significant figures.
please help! will give brainliest.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, marcusajns
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1



Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1
Do you know the correct answer?
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
compute the theoretical yield of the product (in
Questions in other subjects:

English, 24.04.2020 04:58

Spanish, 24.04.2020 04:58


Mathematics, 24.04.2020 04:58




Physics, 24.04.2020 04:58

Physics, 24.04.2020 04:58

Mathematics, 24.04.2020 04:58






