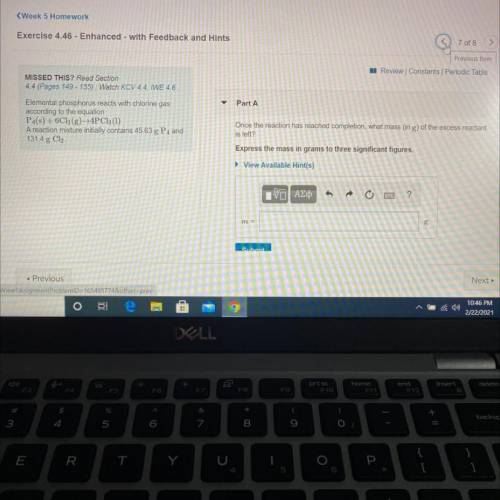
Elemental phosphorus reacts with chlorine gas
according to the equation
P4(s) + 6C12(g)+4PC13...

Chemistry, 23.02.2021 09:00, araminaara691
Elemental phosphorus reacts with chlorine gas
according to the equation
P4(s) + 6C12(g)+4PC13 (1)
A reaction mixture initially contains 45.63 g P4 and
131.4 g Cl.
Once the reaction has reached completion, what mass (in g) of the excess reactant is left?
Express the mass in grams to three significant figures.
please help i’ll give brainliest

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 08:30, aaliyahnv07
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1

Chemistry, 23.06.2019 10:00, JuanTorres7
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 19.11.2020 19:20

Health, 19.11.2020 19:20

English, 19.11.2020 19:20


Arts, 19.11.2020 19:20

Mathematics, 19.11.2020 19:20

Mathematics, 19.11.2020 19:20








