
Chemistry, 23.02.2021 07:10, chrisxxxrv24
HELPP!!Pick one substance and compare two of the atoms connected to each other.
1.Are they close or far away from each other on the periodic table?
2.So they have similar atomic radii?
3.Do they have a large or small difference in ionization energy?what about for electron affinity?
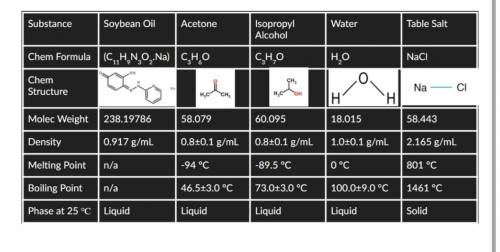

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2


Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
Do you know the correct answer?
HELPP!!Pick one substance and compare two of the atoms connected to each other.
1.Are they close or...
Questions in other subjects:

English, 30.03.2021 17:20



Mathematics, 30.03.2021 17:20


Biology, 30.03.2021 17:20

Mathematics, 30.03.2021 17:20

English, 30.03.2021 17:20


Mathematics, 30.03.2021 17:20






