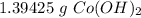Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2Na...

Chemistry, 22.02.2021 23:30, Uhmjujiooo4220
Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2NaCl + Co(OH)₂
If you react 10.0 mL of 1.5 M CoCl₂ with plenty of NaOH, how many grams of Co(OH)₂ will precipitate out?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, kingaman
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2


Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 01.08.2019 04:00

Health, 01.08.2019 04:00

Arts, 01.08.2019 04:00



Mathematics, 01.08.2019 04:00


History, 01.08.2019 04:00


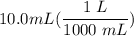 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 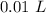 [DA] Find moles of CoCl₂ [Molarity]:
[DA] Find moles of CoCl₂ [Molarity]: 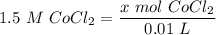 [DA] Solve for x [Multiplication Property of Equality]:
[DA] Solve for x [Multiplication Property of Equality]: 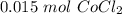 [DA] Set up [Reaction Stoich]:
[DA] Set up [Reaction Stoich]: 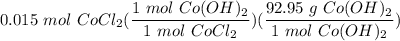 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: