
Chemistry, 22.02.2021 22:50, moisescab662
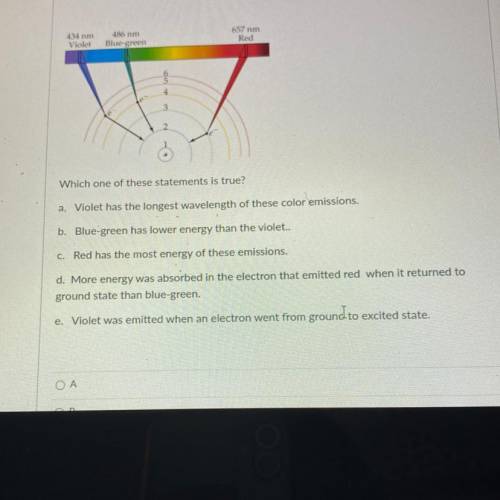
Which one of these statements is true?
a, Violet has the longest wavelength of these color emissions.
b. Blue-green has lower energy than the violet..
c. Red has the most energy of these emissions.
d. More energy was absorbed in the electron that emitted red when it returned to
ground state than blue-green.
e. Violet was emitted when an electron went from ground to excited state.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3
Do you know the correct answer?
Which one of these statements is true?
a, Violet has the longest wavelength of these color emission...
Questions in other subjects:

Business, 19.12.2020 01:40

Mathematics, 19.12.2020 01:40





Mathematics, 19.12.2020 01:40

History, 19.12.2020 01:40

History, 19.12.2020 01:40







