

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2


Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2

Chemistry, 23.06.2019 08:30, brittsterrr
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
Do you know the correct answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions in other subjects:





Mathematics, 21.11.2019 21:31





Mathematics, 21.11.2019 21:31


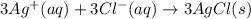
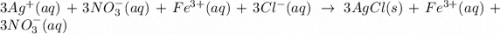
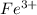 and
and 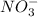 are the spectator ions.
are the spectator ions.




