
Chemistry, 30.09.2019 03:30, ConfusedJuliana
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775 ml of water. calculate the following:
a) moles of k2so4
b)millimoles of k2so4
c)molarity of k2so4, k+, so4(2-)
d)ppm of k2so4
e)%(w/v) k2so4
f)pk+
g)pso4(2-)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
Do you know the correct answer?
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775...
Questions in other subjects:

Business, 02.02.2021 19:50


Social Studies, 02.02.2021 19:50

Mathematics, 02.02.2021 19:50

Arts, 02.02.2021 19:50



Biology, 02.02.2021 19:50

Social Studies, 02.02.2021 19:50


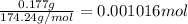
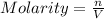
![[K_2SO_4]=\frac{0.001016 mol}{0.775 L}=0.001311 mol/L](/tpl/images/0275/4296/7b54d.png)
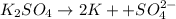
![[K^+]=2\times [K_2SO_4]=2\times 0.001311 mol/L=0.002622 mol/L](/tpl/images/0275/4296/87b7a.png)
![[SO_4^{2-}]=1\times [K_2SO_4]=1\times 0.001311 mol/L=0.001311 mol/L](/tpl/images/0275/4296/fd0e3.png)
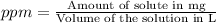
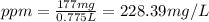
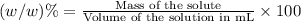
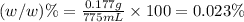
![pK^=-\log[K^+]](/tpl/images/0275/4296/a9ce4.png)
![pK^+=-\log[0.002622 M]=2.58](/tpl/images/0275/4296/0acce.png)
![pSO_4^{2-}=-\log[SO_4^{2-}]](/tpl/images/0275/4296/bd352.png)
![pK^+=-\log[0.001311 M]=2.88](/tpl/images/0275/4296/30697.png)




