
Chemistry, 22.02.2021 18:30, lovelybear2354
You will be provided with the AH values for each equation. These values are state dependent,
meaning that liquid water and steam will have different values. You will need to multiply the
AH, value for each compound by the number of moles of that compound in the balanced
chemical equation, as each value of AH, is for 1 mole of compound. Find the enthalpy of
reaction for the following equation:
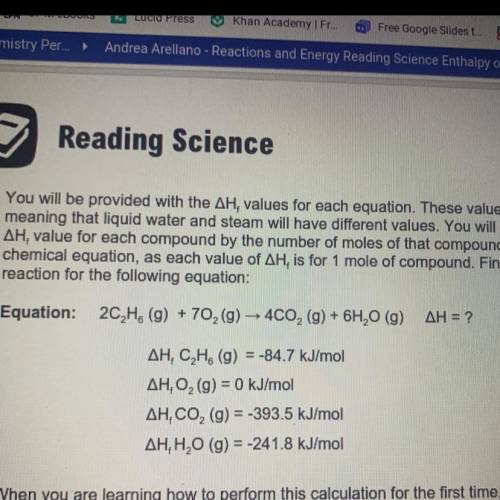

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1
Do you know the correct answer?
You will be provided with the AH values for each equation. These values are state dependent,
meanin...
Questions in other subjects:


History, 05.03.2021 23:00


Mathematics, 05.03.2021 23:00

Business, 05.03.2021 23:00

Mathematics, 05.03.2021 23:00

Mathematics, 05.03.2021 23:00


Geography, 05.03.2021 23:00






