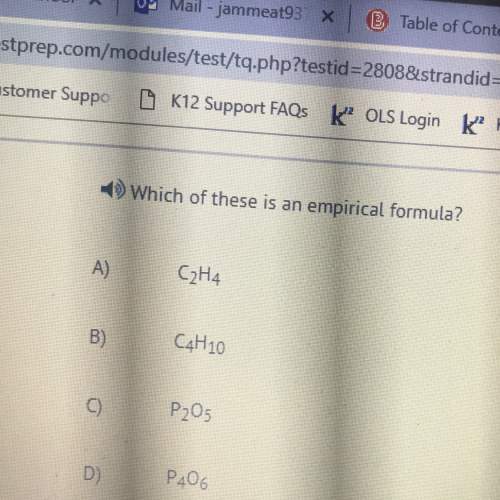
3)5.5 grams of KClO3 breaks apart to KCl + O2
A) how many moles of KClO3 break apart?
B) how...

Chemistry, 22.02.2021 17:10, claudiapineda860
3)5.5 grams of KClO3 breaks apart to KCl + O2
A) how many moles of KClO3 break apart?
B) how many moles of O2 are produced?
C) how many moles of KCl are produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


History, 19.05.2020 14:17



Mathematics, 19.05.2020 14:17

Mathematics, 19.05.2020 14:17

History, 19.05.2020 14:17


Mathematics, 19.05.2020 14:17