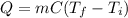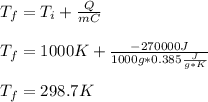Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 06:20, cowboo5000pcl655
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
Do you know the correct answer?
A molten sample of 1.00kg of iron with a specific heat of 0.385J/g. K at 1000.K is immersed in a sam...
Questions in other subjects:

Mathematics, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57

Social Studies, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57

History, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57


History, 20.06.2020 01:57