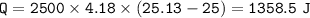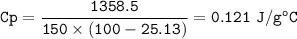Chemistry, 22.02.2021 06:10, lizzyhearts
Use the Gizmo to mix 200 g of granite at 100 °C with 1,000 g of water at 20 °C.
What is the final temperature?
Calculate the temperature change of each substance by subtracting the initial temperature from the final temperature. ∆T water: ∆T granite:
C .How much heat energy (q) did the water gain?
D. Now solve for the specific heat (c) of granite:
E. Repeat steps A through D to find the specific heat (c) of lead:

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
Use the Gizmo to mix 200 g of granite at 100 °C with 1,000 g of water at 20 °C.
What is the final t...
Questions in other subjects:

Mathematics, 30.05.2020 19:59

Social Studies, 30.05.2020 19:59

English, 30.05.2020 19:59

English, 30.05.2020 19:59