Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 23.06.2019 07:00, jboii11
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
Do you know the correct answer?
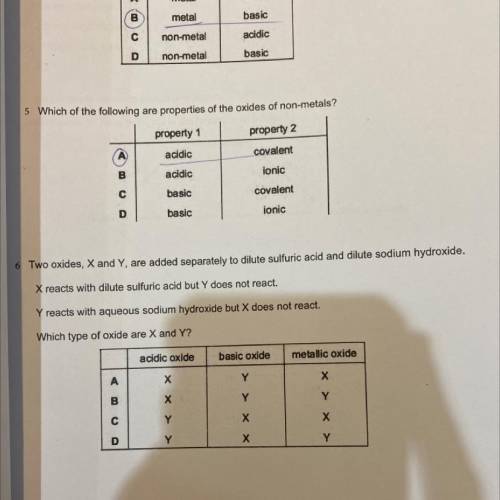
two oxides, X and Y are added seperately to dilute sulfuric acid and dilute sodium hydroxide. X reac...
Questions in other subjects:


Chemistry, 22.10.2020 09:01


English, 22.10.2020 09:01

Mathematics, 22.10.2020 09:01


Medicine, 22.10.2020 09:01