
Chemistry, 21.02.2021 18:00, donaldwilliams31
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aqueous solution of H2S04. (IF = 96500C, Volume at s. t.p = 22.4
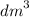

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

Chemistry, 23.06.2019 04:31, saladdressing1425
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
Do you know the correct answer?
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aque...
Questions in other subjects:






Mathematics, 26.06.2020 22:01


Spanish, 26.06.2020 22:01


Mathematics, 26.06.2020 22:01






