
Chemistry, 21.02.2021 03:00, hopelesslylost13
1. 1 N2+ 3 H2→2 NH3 c. How many moles of nitrogen are needed to produce 12 moles of nitrogen trihydride?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, leilanimontes714
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
Do you know the correct answer?
1. 1 N2+ 3 H2→2 NH3
c. How many moles of nitrogen are needed to produce 12 moles of nitrogen trihyd...
Questions in other subjects:

Biology, 26.04.2021 09:40


Mathematics, 26.04.2021 09:40


Geography, 26.04.2021 09:40

Mathematics, 26.04.2021 09:40

German, 26.04.2021 09:40

Mathematics, 26.04.2021 09:40

Mathematics, 26.04.2021 09:40


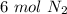
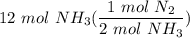 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 




