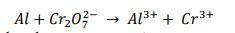Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3


Chemistry, 23.06.2019 01:00, crysderria
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 03:20, elijahmoore841
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
Do you know the correct answer?
1. Consider the equation below:
a) Indicate the oxidation number of the elements that are reactants...
Questions in other subjects:




Mathematics, 07.05.2021 06:00


Mathematics, 07.05.2021 06:00

History, 07.05.2021 06:00

Mathematics, 07.05.2021 06:00