
Chemistry, 19.02.2021 22:30, yaneiryx5476
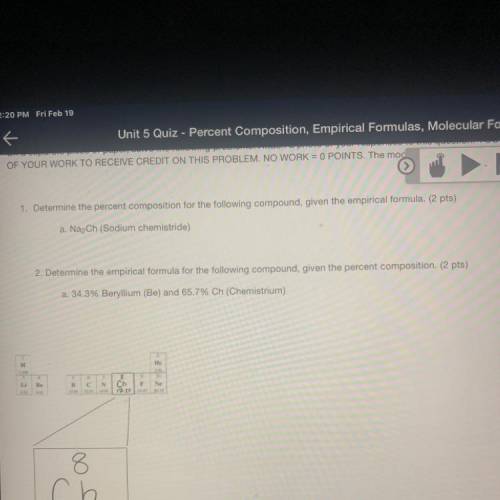
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts)
a. Na2Ch (Sodium chemistride)
2. Determine the empirical formula for the following compound, given the percent composition. (2 pts)
a. 34.3% Beryllium (Be) and 65.7% Ch (Chemistrium)
H
Не
Be
B
c С
N
Ch F Ne
17.250 30
8
ch
Chemistrium
17.25

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


Do you know the correct answer?
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts...
Questions in other subjects:

Computers and Technology, 30.07.2019 04:10














