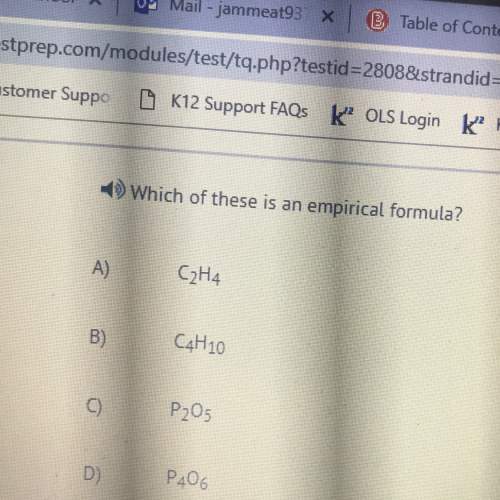
Someone honestly please help me and no trolling i'm really confused and need real help
Take the reaction: 2NH3 + 2O2 --> 2NO + 3H2O. In an experiment, 3.25 g of NH3 are allowed to react with 3.50 g of O2.
a) Which reactant is the limiting reagent?
b) How many grams of NO are formed?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 23.06.2019 00:20, cmflores3245
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
Do you know the correct answer?
Someone honestly please help me and no trolling i'm really confused and need real help
Take the rea...
Questions in other subjects:

Chemistry, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Biology, 13.09.2020 16:01

History, 13.09.2020 16:01

Business, 13.09.2020 16:01

English, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

English, 13.09.2020 16:01

Mathematics, 13.09.2020 17:01

Chemistry, 13.09.2020 17:01