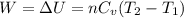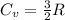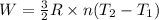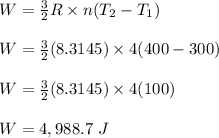Chemistry, 19.02.2021 05:00, helpmewithmath70
4 moles of monoatomic ideal gas is compressed adiabatically causing the temperature to increase from 300 K to 400 K. Calculate the work done on the gas in units of Joules (if the answer is negative, be sure to enter a negative sign in your answer).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, qwerty8364
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 21.06.2019 20:30, hoytkeke6776
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Do you know the correct answer?
4 moles of monoatomic ideal gas is compressed adiabatically causing the temperature to increase from...
Questions in other subjects:









Mathematics, 22.01.2021 18:20

Mathematics, 22.01.2021 18:20